Abstract
Magnesium is essential for the proper functioning of all human cells and is involved in the regulation of neurotransmitter function and neurological function. Acute and chronic hypomagnesemia cause severe neurological symptoms such as neuromuscular irritability, myoclonus, stridor, dysphagia, mainly postural tremor and movement disorders along with vertical downbeat nystagmus. Here we report a case of cerebellar downbeat nystagmus syndrome induced by acute hypomagnesemia (HICS) due to very frequent vomiting in a 75-year-old woman with benign paroxysmal positional vertigo (BPPV). The clinical condition improved with daily intravenous and then oral magnesium supplementation. To our knowledge, this is the first described case of HICS induced by acute hypomagnesemia due to vomiting triggered by a benign peripheral pathology such as BPPV which was then successfully treated once the central syndrome was resolved.
Author Contributions
Academic Editor: Sandra Grumelli, CIMER Universidad Catolica of Cordoba, Argentina.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2024 Vincenzo Marcelli, et.al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have no conflict of interest to declare.
Citation:
Introduction
Magnesium, the second most important intracellular cation after potassium1, is essential for the proper functioning of all human cells and is involved, among other processes, in over 600 enzymatic reactions, including energy metabolism, protein synthesis, stabilization of vascular endothelium and regulation of neurotransmitter function 2, 3, 4, 5, modulating any activity mediated by intracellular calcium concentration fluxes6. In addition, magnesium is involved in the process of myelination7 and in the formation and maintenance of synapses8, making it an element necessary to keep neurons healthy and viable9. Finally, magnesium is an activator of Sirtuin 1, an anti-aging gene important to neurodegeneration and diabetes 10, 11, 12.
Magnesium homeostasis is tightly regulated by small intestinal absorption and renal excretion, and the normal plasma magnesium concentration is 1.8 to 2.5 mg/dL. Although often underdiagnosed13, magnesium deficiency, defined as a serum magnesium concentration < 1.8 mg/dL (< 0.70 mmol/L), is commonly found in 10% of patients admitted to a geriatric facility and up to 60% in intensive care units 14, 15, 16, 17.
The clinical picture of hypomagnesemia varies from asymptomatic presentations to complex clinical pictures characterized mainly by symptoms involving the neurological system, including neuromuscular irritability, myoclonus, stridor, dysphagia, mainly postural tremor and movement disorders along with spontaneous and gaze evoked vertical downbeat nystagmus (DBN).
Here we report a case of acute hypomagnesemia-induced cerebellar downbeat nystagmus syndrome (HICS) due to very frequent vomiting in a 75-year-old woman with an underlying benign paroxysmal positional vertigo.
Case Report
Initial evaluation: December 29, 2023
A 75-year-old woman presented to our clinic with a three-day history of positional vertigo and gait instability, nausea, and especially very frequent vomiting. She had a condition of mild metabolic syndrome (type 2 diabetes and hypertension), her medications included metformin and valsartan. Laboratory tests revealed mildly elevated glycemia (125 mg/dL; normal range 65-110 mg/dL) and mild hypomagnesemia (serum magnesium 1.5 mg/dL, normal range 1.8 to 2.6 mg/dL). Brain magnetic resonance imaging (MRI) revealed subcortical atrophy and nonspecific white matter lesions.
Neuro-otologic evaluation revealed a bilateral stationary and persistent apogeotropic nystagmus in the side lateral positions, which was inhibited by visual fixation. Dix-Hallpike and Pagnini-McClure maneuvers were negative for paroxysmal positional nystagmus.
Therefore, the patient did not have positioning vertigo at that time but had some typical signs of a macular-canal integration deficit, possibly due to a reduced inertial load on the macula utriculi after an otoconial fragment detachment, causing the estimation of the direction of gravity to be biased away from true vertical. This finding is very common after the critical phase of positional vertigo and would support a history of canalolithiasis. The negative response to the Dix-Hallpike and Pagnini-McClure maneuvers may be due to otoconial fragment blockage within the canals or temporary spontaneous resolution.
The patient was advised to take cinnarizine-dimenhydrinate combination twice daily for 15 days to control unsteadiness and vertigo and levosulpiride to control nausea and vomiting. Routine laboratory tests, especially electrolytes, were again required. Follow-up was scheduled in 7 days or sooner if there was a change in clinical status.
Second evaluation: January 04, 2024
The patient still presented to us with persistent positional vertigo, nausea, uncontrollable vomiting, very severe and progressive unsteadiness and gait ataxia, bilateral intention tremor of the arms, headache and confusion. At that time, the neuro-otological evaluation showed a different picture, characterized by a very strong spontaneous and omni-positional vertical down beating nystagmus (DBN), more intense in lateral eccentric gaze. The DBN appeared to be stationary, persistent, not inhibited by visual fixation (second evaluation; video 1) and enhanced by the head shaking test. Vestibulo-ocular reflex suppression under fixation was pathological. Smooth pursuit was impaired in vertical and horizontal planes. The severity of the patient’s condition did not allow the performance of positioning maneuvers, namely Dix-Hallpike and Pagnini-McClure. Neurological examination revealed severe difficulty maintaining an upright position, walking with assistance, and dysmetria on the finger-to-nose test. Laboratory tests revealed severe hypomagnesemia (0.2 mg/dL), hypocalcemia (6.6 mg/dL, normal range 8.01-10.4 mg/dL), and hyperphosphatemia (5.3 mg/dL, normal range 2.5-4.5 mg/dL). 24-hour urinary magnesium was 9 mg (normal 73-122). We hypothesized an acute hypomagnesemia-induced cerebellar syndrome (HICS). Daily intravenous supplementation with magnesium sulfate 2.5 g/10 ml for a total dose of 6 g per day was started immediately. The patient was again advised to take cinnarizine-dimenhydrinate combination twice daily for 15 days to control unsteadiness and dizziness, and most importantly levosulpiride to control nausea and vomiting as needed. Follow-up was scheduled in 7 days or earlier if there was a change in clinical status.
Third evaluation: January 11, 2024
At follow-up, the patient again reported positional dizziness, mild unsteadiness, vomiting, and nausea. Overall, the symptoms showed a slow but progressive improvement with magnesium intake. Neuro-otologic evaluation revealed mild spontaneous and omni-positional vertical downbeat nystagmus (DBN). Smooth pursuit was still impaired in the vertical and horizontal planes. In addition, the left Dix-Hallpike maneuver, was positive for paroxysmal positional vertigo of the posterior semicircular canal, suggesting that the otolith mass was now mobile.
The Epley maneuver was performed with immediate effect (third evaluation; video 2). In fact, unsteadiness was reduced and gait was possible without assistance. Mild dysmetria remained in the finger-to-nose test. The intention tremor of the arms was almost completely resolved. Laboratory tests still showed hypomagnesemia (1.1 mg/dL) and hypocalcemia (7.5 mg/dL). Twenty-four-hour urinary magnesium was 9 mg (normal 73-122 mg). A new brain MRI confirmed the same findings as the previous one (Figure 1). Given the clinical improvement, intravenous magnesium sulfate supplementation was discontinued and daily oral magnesium citrate supplementation (1200 mg/d) was started. Cinnarizine-dimenhydrinate was discontinued, while the patient was advised to take l evosulpiride as needed. Follow-up was scheduled in 7 days or earlier if there was a change in clinical status.
Figure 1.Transverse section of brain MRI (T2 Flair) showing absence of cerebellar edema.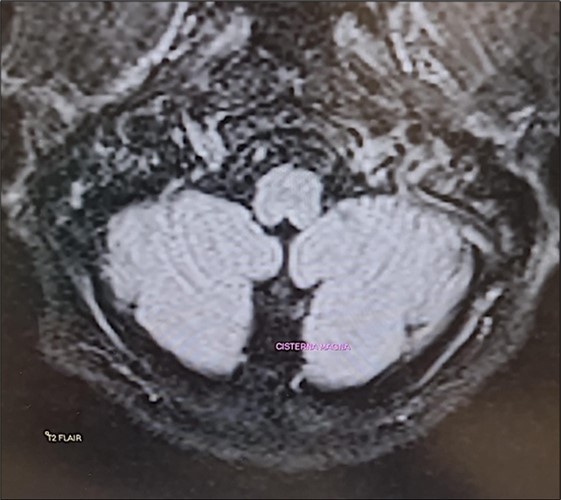
The patient was contacted by telephone and reported significant improvement, so follow-up was postponed for one month.
Fourth evaluation: February 09, 2024
At follow-up, the patient reported only very mild unsteadiness. Neuro-otologic and neurologic examinations were normal, and laboratory tests revealed only very mild hypomagnesemia (1.7 mg/dL). The absence of DBN confirmed the hypothesis of HICS induced by acute hypomagnesemia. Daily oral supplementation with magnesium citrate (800 mg/d) was recommended for ten days until the next follow-up visit, scheduled for three weeks.
Last evaluation: March 01, 2024
The patient was asymptomatic (last evaluation, Video 3). Magnesemia was normal (2.1 mg/dL). All drugs were discontinued.
Discussion
We describe a case of benign paroxysmal positional vertigo in which we hypothesized that hypomagnesemia due to uncontrollable vomiting led to severe hypomagnesemia-induced cerebellar syndrome (HICS) among whose signs was an impressive DBN. Our hypothesis was confirmed by the effective response to magnesium therapy within two months.
The causes of hypomagnesemia can be broadly classified into three categories18: decreased uptake, redistribution from the extracellular to the intracellular compartment, and increased renal or gastrointestinal losses. In our case, it was a magnesium gastrointestinal loss due to neurovegetative reactions from vestibular dysfunction.
The most commonly used laboratory test to assess magnesium status is serum magnesium concentration. Other clinical laboratory tests include measurement of serum ionized magnesium concentration and twenty-four-hour urinary magnesium excretion. Research tests include the magnesium retention test, red blood cell magnesium concentration, and tissue magnesium concentration.
If no cause is readily apparent, gastrointestinal and renal losses can be distinguished by measuring 24-hour urinary Mg2 excretion or fractional excretion of Mg2.
Under normal conditions, magnesium blocks NMDA receptors in the cerebellum, resulting in inhibition of calcium influx and reduction of excitability, and stimulates the GABA-A chloride channel19. Magnesium depletion increases the excitatory effect of glutamate, leading to neuronal hyperexcitability4 and GABAergic channel dysfunction. Dysfunctional GABAergic activity in Purkinje cells causes DBN20. In fact, Purkinje cells send inhibitory projections to the part of the superior vestibular nucleus (SVN) that controls only upward eye movement: disruption of GABAergic activity in Purkinje cells leads to disinhibition of the SVN, resulting in slow upward gaze and DBN21.
Another possible mechanism by which hypomagnesemia may cause cerebellar suffering is related to the vulnerability of the vascular autoregulation of the posterior circulation22. Severe hypomagnesemia results in elevated intracellular calcium levels, leading to increased contraction and tone23, increased perfusion pressure, and ultimately cytotoxic damage and endothelial dysfunction with subsequent vasogenic edema24. These lesions resemble the clinical and imaging features of posterior reversible encephalopathy syndrome (PRES) and pre-eclampsia, which are characterized by posterior circulation edema 25, 26.
In the peripheral nervous system, magnesium acts primarily at the neuromuscular junction27, and decreases endplate sensitivity to the action of acetylcholine28. This explains why hypomagnesemia and hypocalcemia cause tremor.
Finally, the effect of hypomagnesemia on cognition may be related to the effect of low magnesium levels on cholinergic transmission and the overactivity of NMDA receptors29, which could affect synaptic transmission, and disrupt the establishment of long-term potentiation, which is essential for learning and memory30.
It is important to note that although the literature almost always reports MR-documented cerebellar edema18associated with hypomagnesemia, we did not observe the same finding in our patient in two consecutive brain MRIs performed approximately 15 days apart. It is possible that, although the authors do not specify the time at which this evaluation was performed in their patients, it was done at a stage in which hypomagnesemia had been present for some time. In our case, however, a very pronounced hypomagnesemia had developed hyperacutely, and perhaps the cerebellar edema described by other authors had not yet had a chance to develop. To the best of our knowledge, the case of a cerebellar syndrome induced by acute hypomagnesemia as a result of a benign and peripheral disease, has not been described.
Conclusion
The case we present is special for the twofold reason that it shows how the most “benign” cause of vertigo can be complicated by massive neurovegetative effects and cause a severe cerebellar syndrome induced by acute hypomagnesemia and also because, unlike previous reports in the relevant literature, a hypomagnesemia-induced cerebellar edema was not highlighted.
Because magnesium is not usually part of the routine electrolyte panel, the likelihood of under diagnosing hypomagnesemia-related disorders is high, and clinicians need to be aware of hypomagnesemia-related disorders and the severity of their consequences.
Acknowledgments
This study would like to thank Erica de Bernardo and Eugenio Martino.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Fawcett W J, Haxby E J, Male D A. (1999) Magnesium: physiology and pharmacology. , Br J Anaesth 83(2), 302-20.
- 2.Cowan J A. (2002) Structural and catalytic chemistry of magnesium-dependent enzymes. , Biometals 15(3), 225-35.
- 3.Stangherlin A, O'Neill J S. (2018) Signal Transduction: Magnesium Manifests as a Second Messenger. , Curr Biol 28(24), 10-1016.
- 4.de Baaij JH, Hoenderop J G, Bindels R J. (2015) Magnesium in man: implications for health and disease. , Physiol Rev 95(1), 1-46.
- 5.Martin K J, González E A, Slatopolsky E. (2009) Clinical consequences and management of hypomagnesemia. , J Am Soc Nephrol 20(11), 2291-5.
- 6.Sanders G T, Huijgen H J, Sanders R. (1999) Magnesium in disease: a review with special emphasis on the serum ionized magnesium. , Clin Chem Lab Med 37(11), 1011-33.
- 7.Seyama T, Kamei Y, Iriyama T, Imada S, Ichinose M. (2018) Pretreatment with magnesium sulfate attenuates white matter damage by preventing cell death of developing oligodendrocytes. , J Obstet Gynaecol Res 44(4), 601-607.
- 8.Sun Q, Weinger J G, Mao F, Liu G. (2016) Regulation of structural and functional synapse density by L-threonate through modulation of intraneuronal magnesium concentration. , Neuropharmacology 108, 426-39.
- 9.Yamanaka R, Shindo Y, Oka K.Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int J Mol Sci. 2019 Jul 12;20(14): 3439. doi: 10.3390/ijms20143439. PMID: 31336935; PMCID: PMC6678825 .
- 10.Martins I. (2016) Anti-Aging Genes Improve Appetite Regulation and Reverse Cell Senescence and Apoptosis in Global Populations. Advances in Aging Research 5, 9-26.
- 11.Martins I. (2016) Magnesium Therapy Prevents Senescence with the Reversal of Diabetes and Alzheimer’s Disease. , Health 8, 694-710.
- 12.Martins I. (2018) Sirtuin 1, a Diagnostic Protein Marker and its Relevance to Chronic Disease and Therapeutic Drug Interventions. , EC Pharmacology and Toxicology 6, 209-2015.
- 13.Whang R, Ryder K W. (1990) Frequency of hypomagnesemia and hypermagnesemia. Requested vs routine. , JAMA 263(22), 3063-4.
- 14.Martin B J, Black J, McLelland A S. (1991) Hypomagnesaemia in elderly hospital admissions: a study of clinical significance. , Q J Med 78(286), 177-84.
- 15.Deheinzelin D, Negri E M, Tucci M R, Salem M Z, da Cruz VM. (2000) Hypomagnesemia in critically ill cancer patients: a prospective study of predictive factors. , Braz J Med Biol Res 33(12), 1443-8.
- 16.Reinhart R A, Desbiens N A. (1985) Hypomagnesemia in patients entering the ICU. , Crit Care Med 13(6), 506-7.
- 17.Ryzen E, Wagers P W, Singer F R, Rude R K. (1985) Magnesium deficiency in a medical ICU population. , Crit Care Med 13(1), 10-1097.
- 18.Kamm C P, Nyffeler T, Henzen C, Fischli S. (2020) Hypomagnesemia-Induced Cerebellar Syndrome-A Distinct Disease Entity? Case Report and Literature Review. , Front Neurol 11, 968-10.
- 19.Poleszak E. (2008) Benzodiazepine/GABA(A) receptors are involved in magnesium-induced anxiolytic-like behavior in mice. , Pharmacol Rep 60(4), 483-9.
- 20.Glasauer S, Stephan T, Kalla R, Marti S, Straumann D. (2009) Up-down asymmetry of cerebellar activation during vertical pursuit eye movements. , Cerebellum 8(3), 385-8.
- 21.Pierrot-Deseilligny C, Milea D. (2005) Vertical nystagmus: clinical facts and hypotheses. , Brain 128, 1237-46.
- 22.Haubrich C, Wendt A, Diehl R R, Klötzsch C. (2004) Dynamic autoregulation testing in the posterior cerebral artery. 10.1161/01.STR.0000120729.99039.B6. Epub , Stroke 35(4), 848-52.
- 23.Sontia B, Touyz R M. (2007) Role of magnesium in hypertension. , Arch Biochem Biophys 458(1), 33-9.
- 24.Santos A F, Sousa F, Rodrigues M, Ferreira C, Soares-Fernandes J. (2015) Reversible cerebellar syndrome induced by hypomagnesemia. , Neurology and Clinical Neuroscience 3(5), 190-191.
- 25.Boulos M I, Shoamanesh A, Aviv R I, Gladstone D J, Swartz R H. (2012) Severe hypomagnesemia associated with reversible subacute ataxia and cerebellar hyperintensities on MRI. , Neurologist 18(4), 223-5.
- 26.Hammarsten J F, Smith W O. (1957) Symptomatic magnesium deficiency in man. , N Engl J Med 256(19), 897-899.
- 28.Del Castillo J, Engbaek L. (1954) The nature of the neuromuscular block produced by magnesium. , J Physiol 124(2), 370-384.
