Abstract
Intravascular papillary endothelial hyperplasia is an exceptional, benign, inflammatory, vascular neoplasm delineating papillary configuration engendered from reactive proliferation of damaged endothelial cells, while being confined to a thrombus. Initially scripted by Pierre Mason in 1923, the tumefaction was denominated as an intra-luminal lesion within an ulcerated, haemorrhoidal vein and designated as “hemangio-endotheliome’ vegetant’ intravasculaire”(1). The neoplasm is additionally nomenclated as Masson’s tumour, Masson’s pseudo-angiosarcoma, endovascularite proliferante thrombopoietique, intravenous atypical vascular proliferation, intravascular angiomatosis, vascular angiomatosis, intravascular endothelial proliferation, reactive papillary endothelial hyperplasia or intravascular papillary endothelial hyperplasia. The papillary neoplasm is associated with deposition of fibrin and thrombotic substances within a painful, ulcerated.
Author Contributions
Academic Editor: Anand Rotte, University of British Columbia, Canada.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Anubha Bajaj
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Preface
Intravascular papillary endothelial hyperplasia is an exceptional, benign, inflammatory, vascular neoplasm delineating papillary configuration engendered from reactive proliferation of damaged endothelial cells, while being confined to a thrombus. Initially scripted by Pierre Mason in 1923, the tumefaction was denominated as an intra-luminal lesion within an ulcerated, haemorrhoidal vein and designated as “hemangio-endotheliome’ vegetant’ intravasculaire”1.
The neoplasm is additionally nomenclated as Masson’s tumour, Masson’s pseudo-angiosarcoma, endovascularite proliferante thrombopoietique, intravenous atypical vascular proliferation, intravascular angiomatosis, vascular angiomatosis, intravascular endothelial proliferation, reactive papillary endothelial hyperplasia or intravascular papillary endothelial hyperplasia. The papillary neoplasm is associated with deposition of fibrin and thrombotic substances within a painful, ulcerated, haemorrhoidal vein. Henschen demonstrated the reactive rather than a neoplastic nature of the condition 1, 2.
The tumour is described as an endothelial cell neoplasm inducing vascular obstruction and tissue necrosis wherein reactive mechanisms of the lesion appear subsequent to thrombus organization and endothelial restoration. Hyperaemia and lymph stasis along with on site production of angiogenic growth factors are implicated in the genesis of the neoplasm3. On account of complex clinical representation, essentially benign papillary endothelial hyperplasia requires segregation from malignant vascular lesions, in order to circumvent misinterpretation and aggressive therapy as demolition surgery or regional irradiation. Cogent determination of papillary endothelial hyperplasia is challenging on account of clinical and radiographic semblance to diverse vascular tumours, especially angiosarcoma. Also, infrequently discerned recurrent papillary endothelial hyperplasia mandates a distinction from angiosarcoma 3, 4.
Disease Characteristics
Typically, tumefaction is denominated within lumen of distended vascular spaces or pre-existing vascular lesions. A predilection for head and neck, trunk, extremities, fingers or hand is denominated, although uncharted neoplastic presence is documented upon eyelid, orbit, masseters, nasal sinuses, parotid gland, mandible, pharynx, thyroid, oral cavity, sino-nasal cavity, foot and renal parenchyma. Spinal cord, skull and base of skull are sites of skeletal involvement3, 4. Sites incriminated within head and neck are cutaneous and subcutaneous tissue of the lip, oral or buccal mucosa, tongue and gingiva. Commonly, head and neck (23%), lower extremity (17%) and fingers (16%) are incriminated. Although exceptional, intraoral sites implicated in decreasing order of frequency, are lower lip, tongue, buccal mucosa and upper lip. Papillary endothelial hyperplasia is rare within intra-cranial location wherein a malignant neoplasm is recapitulated3, 4.
Papillary endothelial hyperplasia comprises of an estimated 2% to 6% of benign and malignant vascular neoplasms of cutaneous and subcutaneous tissue. A slight female preponderance is observed with female to male proportion of 1.2:1. No age of disease emergence is exempt although the condition is common in adults betwixt 30 years to 40 years. Tumour reoccurrence is discerned within almost 15% subjects. Hashimoto et al, in 1983, subdivided the tumefaction into three distinct categories in accordance to tumour genesis. Papillary endothelial hyperplasia is classified contingent to proportionate proliferation of endothelial cells encompassing the thrombus with concomitant venous stasis 4, 5.
Type I represents a “de novo” neoplasm which stems from normal blood vessels. An estimated 56% instances are denominated as a “pure” or ”primary” form which appear de novo within the lumen of dilated vascular spaces, often a vein or an artery
Type II emerges from a pre-existing, vascular process wherein around 40% of “mixed” or “secondary” subcategory of lesions ensue subsequent to focal modifications within preceding vascular lesions such as haemangioma, pyogenic granuloma, haematoma, vascular malformation, aneurysm, arteriovenous malformation, lymphangioma, vascular hamartoma or chronic disease with venous thrombosis.
Type III is an infrequent variant associated with an extravascular location and generally arises from post traumatic haematoma 4, 5.
Approximately 4% of extravascular lesions develop in association with an organizing haematoma. Distinction from angiosarcoma can be challenging. Distant metastasis is absent as the condition is entirely benign3, 4.
Disease Pathogenesis
Of obscure pathogenesis, several mechanisms are proposed for genesis of papillary endothelial hyperplasia
a) An intravascular endothelial cell proliferation ensues with concomitant configuration of papillary architecture which can progress to necrosis and cellular degeneration3, 4.
b) An exuberant endothelial proliferation with papillary formation originates from a thrombus, vascular stasis or perivascular inflammation with consequent engenderment of a pseudo-tumour, essentially derived from accumulation of thrombotic substances. Thus, it may be posited that thrombotic process may be causative in origination of papillary endothelial hyperplasia3, 4.
c) Autocrine mechanism of engendering post traumatic papillary endothelial hyperplasia is brought about by macrophages, which when induced by the thrombus can activate endothelial cell proliferation through enhanced secretion of basic fibroblastic growth factor (FGF) and consequent augmentation of basic FGF, thereby compounding a positive feedback loop of enhanced endothelial proliferation. It is argued that neoplastic growth is directed by endothelial basic fibroblastic growth factor (FGF) which is generated and released by macrophages4, 6.
d) Trauma may be contemplated as an inciting factor for inducing anomalous organization and proliferation of endothelial cells while encompassing a thrombus. Several instances appear following trauma although cogent clinical history is elicited only in around 4% subjects. Trauma is followed by organization of thrombus. Thrombosis precedes articulation of papillary architecture along with deposition of fibrin, factors which act as a substrate for genesis of endothelial hyperplasia4, 6.
e) Hormonal influence can be encountered, thus delineating a neoplastic tendency for incrimination of females4, 6.
Clinical Elucidation
Papillary endothelial hyperplasia exemplifies a sharply defined, gradually progressive, firm, painless or painful, tender, minimally elevated nodule. Discoloured, bluish or reddish, variously hued superimposed cutaneous surface or mucous membrane is discerned6.
A palpable soft tissue mass situated within normal or distended vascular space is delineated. Apart from cutaneous and subcutaneous tissue of aforementioned sites, papillary endothelial hyperplasia can arise from perineurial vasculature and adhere to abutting nerves. The lesion can mimic a neurogenic tumour and can be accompanied by paraesthesia along with a positive Tinel sign across the distribution of neighbouring or implicated nerves 4, 6.
Histological Elucidation
As associated clinical manifestations and radiological features are non specific and simulate various vascular neoplasia such as angiosarcoma, malignant endovascular papillary angioendothelioma, Kaposi’s sarcoma, haemangioma or lymphangioma, cogent histological evaluation is critical and sufficient in discerning the condition6.
Grossly, a well encapsulated, reddish, bluish or grey/white, tense-elastic tumour nodule encompassed within fibro-adipose tissue is enunciated.
On microscopy, superficial squamous epithelial surface is intact. Sub-epithelial connective tissue stroma exhibits slit-like, vascular spaces. Upon extended magnification, multiple, intravascular papillary projections encompassed within a hyalinised stroma are discerned. Centroidal calcification appears in combination with intravascular, papillary endothelial cell proliferation, lined with singular layer of endothelial cells devoid of cytological atypia6, 7.
Characteristically, the vascular neoplasm denominates numerous papillae within blood vessels. Papillae are coated with singular or dual layer of flattened endothelial cells with an encompassing hyalinised, fibrous tissue core. Vascular lumen is distended with thrombosis. Foci of haemorrhage with fibrinous and purulent exudate are discerned. Tumour perimeter depicts inflammatory granulation tissue. Cholesterol clefts and focal reactive bone formation may concur. Extraneous squamous epithelium may be discontinuous and ulcerated. The neoplasm is devoid of features of malignancy4, 6.
Numerous micro-calcifications can be observed within the lesion which may engender vascular occlusion and tissue necrosis6. Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8.
Figure 1 Papillary endothelial hyperplasia elucidating papillary articulations layered with a single layer of endothelial cells and a commingling of fibrinous, thrombotic exudate 10.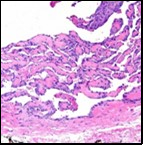
Figure 2 Papillary endothelial hyperplasia delineating papillary articulations with an endothelial cell layer, thrombotic exudate and fibrinous debri (1.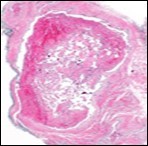
Figure 3.Papillary endothelial hyperplasia exemplifying papillary configuration with endothelial cell layering and a superimposed stratified squamous epithelial lining 12.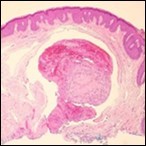
Figure 4.Papillary endothelial hyperplasia enunciating papillary arrangements coated with single layer of endothelial cells intermingled with significant fibrinous and thrombotic exudate13.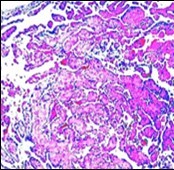
Figure 5.Papillary endothelial hyperplasia exhibiting papillary configurations lined with single endothelial cell layer and lack of significant atypia 14.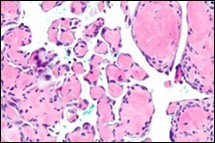
Figure 6.Papillary endothelial hyperplasia delineating papillary articulations layered with a single endothelial cell layer and an admixed fibrinous exudate 15.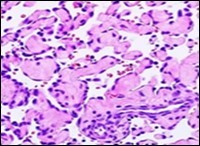
Figure 7.Papillary endothelial hyperplasia demonstrating significant papillary architecture, a single lining of endothelial cells intermixed with fibrinous and thrombotic substances and lack of atypia 16.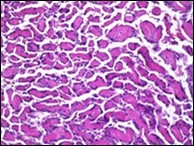
Figure 8.Papillary endothelial hyperplasia depicting papillae layered with a single endothelial layer, absence of atypia and fibrinous, thrombotic material 17.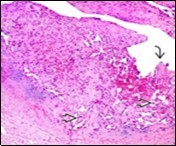
Immune Histochemical Elucidation
Typically, papillary endothelial hyperplasia is immune reactive to CD31 or CD34, sensitive markers indicating vascular origin of the lesion. Tumour cells are immune reactive to vascular endothelial cell marker CD343, 4.
Enunciation of proliferation index Ki-67 during cellular proliferation may indicate a neoplastic origin although accompanying granulation tissue proposes a reactive aetiology of endothelial cell proliferation. Thus, aforesaid premise is contemplated as a likely mechanism for genesis of papillary endothelial hyperplasia3, 4.
Differential Diagnosis
Clinical and radiographic demarcation of papillary endothelial hyperplasia is necessitated from malignant bone tumours such as intraosseous odontogenic carcinoma, clear cell odontogenic carcinoma, ameloblastic fibrosarcoma and osteosarcoma6. On account of non specific clinical manifestations and contingent to location and tumour magnitude, papillary endothelial hyperplasia can simulate diverse lesions such as mucocoele, haemangioma, haematoma, intravenous pyogenic granuloma, phlebectasia, salivary gland tumours, cutaneous nevi, Kaposi’s sarcoma, haemangiopericytoma, angio-endothelioma, papular angioplasia, Kimura’s disease, bacillary angiomatosis , intravenous atypical vascular proliferation, sinusoidal haemangioma and angiosarcoma7.
Sinusoidal haemangioma is an exceptional variant of cavernous haemangioma which commonly appears within subcutaneous tissue of extremities and delineates a female predominance. Histologically, sinusoidal haemangioma is comprised of distended, conjoined, thin walled blood vessels. Pseudo-papillary configurations, layered with endothelial cells, can be observed, thus simulating papillary endothelial hyperplasia. Differentiation betwixt the lesions is obtained from identifying a true papillary pattern, typically demonstrated in papillary endothelial hyperplasia4, 6. Distinction of papillary endothelial hyperplasia from angiosarcoma or adjunctive benign or malignant vascular neoplasms can be challenging. Segregation from angiosarcoma is crucial. Foci of organized thrombus confined to dilated blood vessels and proliferation of endothelial cells configuring a papillary architecture articulate the neoplasm. Also, occurrence of thrombotic substances along with absence of features of malignancy such as nuclear hyperchromasia, cellular pleomorphism, atypical mitosis, foci of necrosis and irregular capillaries aid the distinction betwixt papillary endothelial hyperplasia and angiosarcoma.
Angiosarcoma arising intravascularly within a lumen is an extremely exceptional feature and provides a crucial clue in demarcating the sarcoma from papillary endothelial hyperplasia, except the extravascular variant of type III4, 6.
Histological distinction betwixt papillary endothelial hyperplasia and angiosarcoma is denominated by
1) Endothelial cell proliferation is confined within the vascular lumen, in contrast to angiosarcoma, where cellular proliferation is rarely intravascular or restricted to vascular lumen as neoplastic cells tend to invade circumscribing soft tissues and exhibit an infiltrative pattern of tumour evolution
2) Lack of necrosis, absence of cellular pleomorphism or atypical mitosis
3) Majority of papillary articulations are associated with thrombi Intracranial papillary endothelial hyperplasia is associated with significant morbidity and necessitates distinction from mass lesions treated with gamma knife radiosurgery4, 6.
Investigative Assay
Ultrasonography demonstrates associated singular or multiple blood vessels, thus differentiating papillary endothelial hyperplasia from adjunctive soft tissue nodules. On ultrasonography, papillary endothelial hyperplasia appears as a well defined, confined or expansive, echogenic mass. The mass can be incorporated intramuscularly or appears within peripheral vein, endovascular thrombus or subcutaneous tissue of implicated site7, 8.
Colour Doppler sonography exemplifies a hyper-vascular lesion with combination of arterial and venous flow. Due to diverse representations, computerized tomography (CT) with intravenous contrast media and magnetic resonance imaging (MRI) delineate the vascularity and extent of lesion although may not be efficacious in differentiating the neoplasm from adjunctive vascular conditions7, 8
Magnetic resonance imaging (MRI) depicts a minimally heterogeneous mass, isointense as compared to muscle, on T1 weighted imaging whereas T2 weighted imaging displays a centrally heterogeneous, isointense mass or minimally enhanced signal intensity. The mass is completely or incompletely circumscribed by peripheral zone of enhanced signal intensity. Post contrast T1 weighted imaging delineate heterogeneous image enhancement8.
T1 weighted imaging sequences can be hypo-intense with a heterogeneous signal on account of intra-lesional haemorrhage. Upon T2 weighted imaging, the mass appears hyper-intense with minimal signal intensity due to internal septa. Hypo-intense areas are indicative of haemorrhage or accumulation of thrombotic material. Diffuse enhancement can also be observed on MRI7, 8.
Therapeutic Options
Comprehensive surgical extermination of the lesion is optimal, curative and is accompanied by an excellent prognosis. Surgical resection with appropriate tumour-free perimeter is controversial as removal of wide margin or normal tissue is pertinent to configuration and location of the neoplasm8, 9. Tumour relapse can ensue with inadequate surgical extermination or with co- existent vascular tumour. Proportionate tumour reoccurrence is minimal, especially in type II8, 9.
Radiotherapy can be therapeutically employed although indications are obscure. Radiotherapy can be adopted for alleviating partially excised, recurrent neoplasm encasing a neurovascular bundle with concomitant preservation of neurovascular bundle and is associated with superior outcomes and absence of tumour reoccurrence9.
Sclerotherapy with sodium tetradecyl sulphate prior to surgical resection can assist in minimizing haemorrhage and enhancing cosmetic outcomes9. Therapeutic measures such as endoscopic surgery and employment of beta- adrenergic antagonists are contingent to site of tumefaction.
Multiple intracranial lesions or anatomic limitations to surgical intervention with consequent incomplete surgical extermination can be managed with adjuvant radiotherapy or chemotherapy with favourable outcomes9.
Adjuvant radiotherapy or chemotherapy can be employed for eradicating remnant lesions following inadequate resection. Multiple, intracranial lesions can be subjected to radiotherapy or chemotherapy which stabilizes the lesion and ensures short- term retrogression. Although exceptional, tumour relapse is documented, subsequent to incomplete tumour extermination or reappearance of primary vascular lesion. Fatal clinical outcomes may ensue along with intracranial haemorrhage 8, 9. Table 1.
Table 1. Papillary Endothelial Hyperplasia versus Angiosarcoma (3).| Papillary Endothelial Hyperplasia | Angiosarcoma | |
| Nature | Rare, benign, vascular neoplasm | Malignant, aggressive soft tissue sarcoma |
| Location | Extremities, head and neck | Skin, scalp, breast, liver, spleen, deep-seated tissues |
| Cause | De novo, post-traumatic haematoma, vascular injury | Lymph-oedema, radiation, poly-vinyl chloride, arsenic, thorium dioxide |
| Clinical Presentation | Well defined superficial papules or deep nodules. Progressive nodule with discoloration of superimposed skin | Bruise-like patches, violaceous nodules, plaques, enlarged, painful mass |
| Magnetic resonance imaging | Minimally heterogeneous on T1 and centrally heterogeneous, high signal intensity on T2, complete or incomplete peripheral high signal intensity | Intermediate T1 signal intensity with possible hyper-intensity, indicating haemorrhage. High T2 signal intensity, enhanced with intravenous contrast |
| Histopathology | Hyperplastic endothelial cells with an intravascular, intra-luminal papillary pattern | Subcutaneous infiltration, papillary endothelial hyperplasia, prominent nucleoli, mitosis, cytological atypia, dissection of dermal collagen |
| Immune Histochemical reactions | CD31+, CD34+, SMA+, Factor VIII-related antigen + | CD105- |
| Management | Local excision is curative. Radiotherapy controversial | Surgery, radiotherapy, chemotherapy |
| Metastasis | None | Nodal and distant metastasis- poor prognosis |
| Tumour Reoccurrence | Rare | Frequent loco-regional recurrence |
References
- 1.Masson P. (1923) He’mangioendothe’liomex ve’ge’tant intravasculaire Bull Soc Anat. , Paris: 93, 517-523.
- 2.Henschen F. (1932) L’endovasculite proliferante’ thrombopoitique’ dans la lesion vasculaire locale”. , Ann Anat 9, 113-121.
- 3.Almarghoub M A, Mardan Qutaiba NM Shah. (2020) Masson’s tumour involving the hand- a case report”. , Int J Surg Case Rep 70, 223-226.
- 4.Shim H K, Kim M R. (2019) Intravascular papillary endothelial hyperplasia of the vocal cord- a case report and review of literature”. , Am J Case Rep 20, 1664-1668.
- 5.Hashimoto H, Daimaru Y. (1983) Intravascular papillary endothelial hyperplasia- a clinicopathologic study of 91 cases”. , Am J Dermatopathol 5(6), 539-546.
- 6.Mirmohammdsadeghi H, Mashhadiabbas F. (2019) Huge central intravascular papillary endothelial hyperplasia of the mandible - a case report and review of literature. , J Korean Assoc Oral Maxillofac Surg 45(4), 180-185.
- 7.Pesce V, Bizzocca D. (2018) An intravascular papillary endothelial hyperplasia of the hand radiologically mimicking a hemangiopericytoma- a case report and literature review” SAGE Open Med Case Rep.6:.